Outline of the Pandas Chapter |
|
General:
This chapter tries to discredit all the morphological evidence for evolution. It tries to convince us that homology is a uniquely evolutionary concept based on evolution and totally subjective so that evolutionary classifications and relationships are based on circular reasoning.
Introduction: Historical
Louis Agassiz, (1807-1873) the well-known 19th century creationist zoology/geologist wrote in his 1857 Essay on Classification (Davenport, 1983; pp. 45, 46, 104):
"Nothing is more striking throughout the animal and vegetable kingdoms than the unity of plan in the structure of the most diversified types."...
"During the first decade of this century, naturalists began to study relations among animals which had escaped almost entirely the attention of earlier observers. Though Aristotle knew already that the scales of fishes correspond to the feathers of birds, it is but recently that anatomists have discovered the close correspondence which exists between all the parts of all animals belonging to the same type, however different they may appear at first sight. Not only is the wing of the bird identical in its structure with the arm of man, or the fore leg of a quadruped, it agrees quite as closely with the fin of the whale, or the pectoral fin of the fish, and all these together correspond in the same manner with their hind extremities. Quite as striking a coincidence is observed between the solid skull-box, the immovable bones of the face and the lower jaw of man and the other mammals, and the structure of the bony frame of the head of birds, turtles, lizards, snakes, frogs and fishes. But this correspondence is not limited to the skeleton; every other system of organs exhibits in these animals the same relations, the same identity in plan and structure, whatever be the difference in the form of the parts, in their number, and even in their functions. Such an agreement in the structure of animals is called their homology, and is more or less close in proportion as the animals in which it is traced are more or less nearly related."
"Embryology has, however, a wider scope than to trace the growth of individual animals, . . . it ought also to embrace a comparison of these forms and the successive steps of these changes between all the types of the animal kingdom, in order to furnish definite standards of their relative standing, of their affinities, of the correspondence of their organs in all their parts."
The discovery of these underlying similarities was used by the 19th century morphologists to bring order into the living universe and gave rise to the idealistic morphology of the German Naturphilosophen. J. W. Goethe (1749-1832), the German poet and intellectual who also did vertebrate dissections asserted that ". . . are all formed according to an Urbild (Archetype) which varies only more or less in its basically constant parts . .." (Mayr, 1982). The renowned French morphologist Cuvier (1769-1832) recognized four great archetypes: vertebrates, articulates (annelids and arthropods), molluscs and radiata (the remaining invertebrates). The English anatomist, Richard Owen (1804-1892) who worked on the Archetype and homologies of the vertebrate skeleton, (Mayr, 1982; Gould, 1986b) distinguished between analogies (similar of function) and homologies:
"Analogue: a part or organ in one animal which has the same function as another part or organ in a different animal."
"Homologue: the same organ in different animals under every variety of form and function."
Homologies were identified by means of the principle of connections of Geoffroy Sainte-Hilaire (1772-1844). When in doubt of the homology of structures in widely different organisms, say a fish and a mammal, "the sole general principle one can apply is given by the position, the relations, and the dependencies of the parts . . ." (Mayr, 1982).
Richard Owen was the last serious representative of morphologists looking for the real essence, the ideal type (Urform); unity of plan; and a limited number of archetypes. This however, was a radical departure from orthodox natural theology, where each structure designed purely for the sake of utility for a particular species. "But then why should the anterior extremity of a mole (digging tool), a bat (wing), a horse (running leg) and a whale (paddle) have essentially the same structure, while the wings of insects, birds and bats, all serving the same function, have very different structures?" The Archetype principle was a deistic mode of ascribing structure to natural laws; it had primarily esthetic satisfaction, being devoid of explanatory capacity. In fact, the idealistic morphologists were completely as a loss to explain the unity of plan and, more particularly, why structures rigidly retained their pattern of connections no matter how the structures were modified by functional needs (Mayr, 1982).
"Nothing can be more hopeless than to attempt to explain this similarity of pattern in members of the same class, by utility or by the doctrine of final causes. The hopelessness of the attempt had been expressly admitted by Owen in his most interesting work on the ‘Nature of Limbs.' On the ordinary view of the independent creation of each being, we can only say that so it is; — that it has so pleased the Creator to construct each animal and plant." (Darwin, 1968, p. 414).
The Modern Interpretation
Only on the assumption of descent with modification did the complex combination of unity of plan and adaptive variety fall into place! Natural selection can only select modifications that are functional and of adaptive value to an organism. It follows that any "new" organ or structure must be derived by modification of some already existing organ or structure either though an intensification or shift in its function (Mayr, 1963, p. 602 fol.).
Although homology has now been redefined: "Attributes of two organisms are homologous when they are derived from an equivalent character of the common ancestor." (Mayr, 1982), the determination of homologies follows from Geoffroy's principle of connections, embryology, fossil sequences and more recently from biochemical and genetic similarities (Gould, 1988). The more detailed the similarities between two structures the less likely that they are the result of parallel or convergent evolution and the more likely that they are derived from a common ancestor. Thus, homologies are determined and evaluated by evolutionary biologists employing exactly the same criteria as used by the 19th century pre-evolutionary anatomists. Simpson (1961) devotes an entire chapter to Taxonomic Evidence and Evolutionary Interpretation, including the criteria for homology.
Homologies of all the anatomical parts of vertebrates, including bones, teeth, muscles, nerves, heart and blood vessels and internal organs are pretty definitely settled, most of the work having been done by pre-evolutionary biologists. A summary of this information is given by Goodrich (1958). Some of the problems, such as the pandas and various bird orders (ex: flamingoes, Gould, 1985) involve homologies of the adaptive modifications of these parts. Modern techniques, such as DNA-DNA hybridization, promise to solve these problems, as was recently done with the birds. Australian birds show a picture similar to the marsupials and placentals, with many ecological equivalents to birds in other parts of the world. The DNA-DNA hybridization technique indicates that these are cases of parallel evolution, as are the marsupials.
Pandas' implication that evolutionists recognize different homologies than did the pre-evolutionary biologists is false. All the 19th century biologists considered human hands and dogs' paws homologous. Owen, in fact, tried to homologize the skeletal elements of all vertebrates in terms of one Archetype (Gould, 1986b). Pandas' assertion that no continuously evolving series of fossils have ever been found is false. This has been discussed with regard to Excursion chapter 4. Pandas' point that some evolutionists are skeptical about the value of fossils in determining phylogenies is misleading, to say the least. Footnote 1 refers to Patterson (1981) and Forey (1982). Patterson concludes that the belief that fossils are the only or best means of determining evolutionary relationships is a myth because he has not found any instances of fossils overturning theories of relationship based on recent organisms (Patterson, 1981, p. 218). Patterson is a cladist and cladists prefer to construct classifications using data on the morphology, physiology, embryology, biochemistry, etc. of living forms. Forey (1982) distinguishes between the philosophies behind cladograms versus phylogenetic trees, using brachiopods (the great majority of which are known only as fossils!) as an example.
Homology Or Analogy: A Problem Of Interpretion (Sic)
The Meaning Of Analogy
Structures may look different and function differently, but still be considered homologous, not to "satisfy" evolutionary theory, but because their morphological interconnections (Geoffroy's principle of connections), embryological similarities, etc. warrant that conclusion (see above). Structures having a similar appearance and function in unrelated groups are analogous. Furthermore, they usually differ in their morphological details, as do, for example, the skeletal structure of the wings of bats, birds and pterosaurs.
The Marsupials
The early workers were unaware of the great gulf between the reproductive and other systems of the monotremes (discovered about 1790), the marsupials (opossums discovered in the sixteenth century and the Australasian forms about 1760), and the placentals (the only mammals really well-known until the nineteenth century), and thus the basis for primary subdivision of the Mammalia was unlike that now universally accepted. The eighteenth century naturalists were invariably misled by the convergence of marsupials with different placentals. The unity of the Marsupialia was finally noted by De Blainville (in 1816) who united them as "didelphes normaux" and proposed three subclasses of mammals: the monotremes, marsupials and placentals (Simpson 1945, p. 164, 170). Thus the Marsupialia are not just a whim of the evolutionists. The group was originally created by pre-evolutionary biologists.
Besides their reproductive system and embryology, marsupials share many details of skull, teeth and skeleton. Paleontologists can identify marsupial fossils by these means and any good anatomist can distinguish a skull of a dog from that of a tasmanian wolf (Carroll, 1988, p. 430). All of the resemblances between various marsupials and placentals are adaptations related to various similar ecological ways of life. Consideration of anatomy, physiology, cytology, electrophoretic and serological studies all indicate that the marsupials are a natural taxon (Archer, 1984).
The Dog, The Wolf And The Thylacine (Tasmanian Wolf)
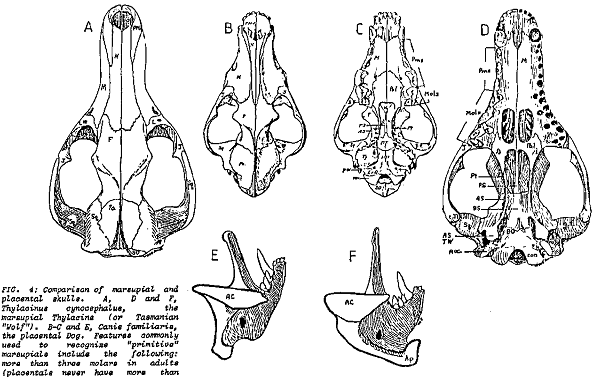
In the caption to Pandas' Figure 5-2, it is claimed that the wolf skull is nearly identical to that of the Tasmanian wolf and much less similar to that of the dog. The accompanying text claims that the two wolves are "superficially almost identical." Actually, by looking carefully at the drawings of the three skulls, it is obvious that the dog and wolf share more specific features that the wolf and the Tasmanian wolf. One of the convergent similarities of the two forms is the carnassial teeth, the broad blade-like teeth in the upper and lower jaws that acts like scissors to slice flesh. In the wolf and dog (as in all placental carnivores) it is the last upper premolar and the first lower molar that are so modified. The other molars are reduced in size and act as crushing teeth. In contrast it is the last four molar teeth in both jaws of the Tasmanian wolf that are modified as carnassials. Clearly the carnassials of placental carnivores and the Tasmanian wolf are not homologous. In addition, the skull of the Tasmanian wolf has four molars (placentals never have more than three), only three premolars (placentals have up to four), holes in the palate, posteriorly expanded nasal bones, an alisphenoid tympanic wing flooring the middle ear, the involvement of the jugal at the edge of the glenoid fossa for articulation of the lower jaw, broad extension of the lachrymal bone onto the face of the skull and mesially enlarged angular process of the dentary (lower jaw), features which it shares with most other marsupials (Archer, 1984). In addition, the teeth appear to be homologous to the placental milk teeth; the only marsupial tooth that is replaced in life is the third premolar. Taking all these characters together, anyone can easily distinguish between the skulls of a wolf and thylacine (Figure 5.1). Denton's claim (Denton, 1986, p. 178) that only a skilled zoologist can distinguish them is nonsense.
Of course, the Tasmanian wolf has the reproductive anatomy, physiology and embryogeny characteristic of all marsupials. Like all other marsupials, it has a relatively small brain with no corpus callosum connecting the two cerebral hemispheres and exhibits no pack or herd organization (Bergamini, 1964, pp. 80, 84). At most it hunted in pairs. Finally, serological tests based on albumen recovered from dried museum skins (the Tasmanian wolf is extinct, the last known live specimen died in the Hobart zoo in 1934) indicate that it is closely related to another groups of Australian marsupials, the dasyurids (Archer, 1984; Sarich et al, 1982). In general proportions it is similar to dasyurids and differs from the wolf in virtually all structural features relating to the pursuit carnivore role (Keast, 1982). The live Tasmanian wolf, with its striped back, long tapered tail and relatively short legs, looked like a strange wolf indeed (see Park, 1985). All biologists, including the pre-evolutionary 19th century anatomists classified the Tasmanian wolf as a marsupial. Pandas gives the impression that there is something wrong with this classification—that it is an arbitrary evolutionist whim—but never explicitly gives an alternate creationist view.
The Panda Connection
The similarities of the Giant and Lesser Pandas are related to their similar ecologies (feeding on bamboo). The fact that their ancestors, bears and racoons, are themselves relatively closely related, made the analogies and homologies difficult to untangle (see Gould, 1986a; Mayr, 1986; O'Brien, 1987 for a historical review). Footnote 2 (p. 118) refers to Davis (1964). But what of the Panda's thumb (Pandas' Figure 5-6 on p. 120 is somewhat distorted. Compare it with the diagram in Gould, 1978 or Gould, 1980, p. 22). If an intelligent designer is indeed working from a small vocabulary of forms (Pandas, p. 133), why didn't it give the panda an efficient opposable thumb as it did the Primates?—instead of the marginally efficient enlarged radial sesamoid bone! The lesser panda doesn't have a "thumb" (Mayr, 1986, p. 770). Similarly, a really intelligent designer would have endowed the panda with enzymes to digest cellulose and lignin. As it is, the pandas digestion is only 17% efficient and individuals must spend 10 to 12 hours a day eating in order to process enough bamboo to sustain themselves (Gould, 1980; Schaller et al, 1989). (Similarly the intelligent designer would have given cows such enzymes instead of the complex stomach and complex bacterial fermentation process that they actually have!) Incidentally, Pandas' Figure 5-5 portrays the undersides of several skulls (which include the upper jaws), not just the upper jaws themselves!
Three different and independent molecular techniques (DNA-DNA hybridization, isozyme genetic distance based on more than 50 loci and immunological distance of serum proteins support the conclusion that the giant panda is related to the bears (O'Brien et al, 1985; O'Brien, 1987). Bears have 74 acrocentric chromosomes (the centromere is at one end) while the giant panda has 42 large metacentric (the centromere is in the middle) chromosomes each with two arms. Although the chromosome count for the giant panda is closer to that of the lesser panda (Pandas, p. 119, bottom), using G-trypsin banding technique, nearly every bear chromosome could be aligned with a giant panda chromosome arm, whereas only two of the giant panda or bear chromosomes had recognizable counterparts in the lesser panda or racoon. On the other hand 14 chromosomes of the lesser panda were strikingly homologous to chromosomes found in several procyonids (O'Brien et al, 1985; O'Brien, 1987).
 |
The biochemical evidence indicates that the lesser panda is related to the Procyonidae (racoon, etc.) which, in turn, is the family closest to the Ursidae (the bears). Figure 5.2 summarizes these relationships in a phylogenetic tree. Probably the morphological similarities of the two pandas result from the parallel retention of ancestral characters that may have been later lost by the bears (O'Brien et al, 1985; Wayne et al, 1989). The lesser panda may occupy an intermediate position between the procyonids and ursids (Wozencraft, 1989a, p. 515; Wozencraft, 1989b, p. 579). Although Pandas points out that the giant panda doesn't hibernate, that doesn't mean very much. The only bears that hibernate are the black, brown and grizzly bears of north-temperate America and Eurasia. The asiatic black bear of southern and eastern Asia may or may not hibernate depending on the severity of the weather; the spectacled bear of South America, the sloth bear of India, the sun bear of southeast Asia, and the polar bear do not hibernate (Walker, 1975). What fossils there are indicate that the giant pandas evolved from the bears and the lesser panda is related to the procyonids (Mayr, 1986).
Linking Classes Together With Homologies
Pandas tries to discredit the homology of the mammalian ear bones and the therapsid jaw joint. This is, in fact, one of the most thoroughly documented and incontrovertible examples of homology (Gould, 1990). It is based on extensive morphological, embryological and fossil data. For a detailed discussion of the similarity of connections of these bones with other bones, muscles, nerves and blood vessels, see Goodrich (1958). The homology was first proposed by the German embryologist Reichert in 1837 (Goodrich, 1958) on the basis of the identical embryological beginnings of the reptilian articular and quadrate bone and the mammalian malleus and incus. In a sense, the marsupial embryo recapitulates the transformation: in the early embryo, the elements serve as a reptilian type jaw joint; when the dentary bone eventually contacts the squamosal bone of the skull and establishes mammalian jaw joint, these elements move into the middle ear and take up the function of the ear bones! There is extensive fossil documentation of the steps leading up to the final transformation among the latest therapsids and the earliest mammals. For more details, see the supplement: The Reptile-Mammal Transition.
Homologies And The Fossil Record
Palaeontological judgments may be more subjective when specimens are incomplete and the animals are quite different from known living forms. Otherwise the identification of individual bones can be quite definite, especially since the differences are due to modifications of the same basic skeletal parts characterizing the vertebrate groups and not to radically different skeletal designs.
Pandas' Footnote 3 relating to fossilized dinosaur eggs is to Horner and Gorman, (1988, pp. 163-165). Paleontologists (evolutionary or not) working on fossil mammals deal mainly with teeth, jaws, skulls and post-cranial skeletal material, but not with "general external anatomy". Were the Pandas' authors half-asleep when they wrote that sentence beginning at the bottom of the first column on p. 122?
According to Pandas, the design proponent explains analogous features on the basis of design requirements. But so does convergent evolution, except that natural selection is responsible for "designing" the structures that meet the requirements for a particular function, not a supernatural creator. If a creator were responsible, we would expect the designs to be much more similar; the flesh-slicing teeth of the placental and marsupial wolves would be formed from the same (homologous) teeth. (Why would such a creator makes two groups of wolves with different reproductive systems? Or for that matter three different types of flying vertebrates? Surely there would be one best design for a wing!) Animals best adapted to flying through the air or swimming in water are those whose structures most closely satisfy aerodynamic and hydrodynamic requirements.
The Concept Of Homology: Limits Of Its Usefullness (Sic)
Subjective Determination Of Homologies Among Living Organisms
Again Pandas brings up a confusing discussion of marsupials versus placentals. We have already seen that there is a great deal of anatomical evidence for separating the two major groups. This was first done by early 19th century pre-evolutionary anatomists, not evolutionists. Would design proponents want to change the classification? Exactly what is their position concerning marsupials? If both creationists and evolutionists agree that there are marsupials and placentals, there must be some objective reasons for making the distinction!
Homology Requires Assumptions
As has already been pointed out, evolutionary biologists empirically determine homologies using the same evidence that pre-evolutionary biologists did. Obviously this does not entail assuming evolution to be true! Pre-evolutionary 19th century biologists created the group Marsupialia based on a large number of homologous structures. Evolutionary biologists explain the existence of those homologies on the basis of common ancestry. No circular reasoning is involved.
The Intelligent Design Alternative
The alternative is that marsupials and placental were designed that way; that the designer decided to have two kinds of mammals (ignoring for the sake of simplicity, the monotremes as a third kind of mammal.) Pandas cannot come up with any reason for the designer's action. The authors admit that the designer's reasons will not be obvious to us, but they claim that such concepts will generate scientific research. What possible lines of scientific research can investigate the thoughts and motives of a supernatural creator? None. Intelligent design (creationism) is a completely sterile doctrine. It offers no understanding of the nature of the designer or how it (or they) decide(s) what to design and why or by what processes designs are made into reality. Thus it offers no explanations. The "intelligent design explanation" is the same as "supernatural explanation". Both are oxymorons. They are not explanations but statements saying that explanations are impossible and beyond human understanding. The NASA search for extraterrestrial intelligence and the archaeologist's recognition of the intelligent design of artifacts deal with natural, not supernatural intelligences, which can be investigated by science. All creationists, however, adamantly insist that their creators/designers are supernatural, hence religious mysteries, not scientific explanations.
What Is Homology, Really?
This section does little to answer the question in the section title! It simply reiterates the vague and false criticisms presented earlier. Pandas insist that some homologies are farfetched (ex: the ear ossicles) and cite a number of other alleged difficulties without giving any examples. (If the ear ossicles are not homologous, how do the design proponents explain their embryogeny, especially in the marsupials?)
Homology is the main tool used by taxonomists (including cladists) to create biological classifications. It is certainly not used "very selectively and subjectively, when it can be applied at all." This is just as true of the 19th century pre-evolutionary biologists as it is of evolutionary biologists. The concept can be applied in an objective manner and it is not dependent on evolution. After all, the concept was invented by non-evolutionists. As pointed out at the beginning of this chapter, the early 19th century biologists applied the concept of homology across all vertebrates, not just to those closely related as the design proponents would limit it.
The resolution of the panda relationship question (Davis, 1964; O'Brien et al, 1985; see also Mayr, 1986) was a triumph for the methodology of evolutionary biologists based on the concept of homology. We are not given any alternative method of investigating the question by the design proponents. Presumably the designer/creator(s) made bears, pandas and racoons the way they are because that's the way it/they desired to do it!
It is claimed that chimps and starfishes have no common ancestry but Hawaiian fruit flies do! What objective criteria do the design proponents use to decide such a question? In more traditional creationist terms, how does one go about discovering the boundaries of a kind? How much evolution can a kind display? Pandas sidesteps this critical question on p. 78. Certainly the originally designed kinds would not be artificial human groupings. How much evolutionary modification can go on within a kind? It we can't determine this, and cannot know the possible extent of a kind, how can we decide which animals are related and which are unrelated and when it is legitimate to apply the concept of homology?
Recapitulation: An Evolutionary Application Of Homology
The 19th century morphologists discovered that the early stages of embryological development are much more similar than the adult forms. The facts were generalized in at least two ways: von Baer's laws and recapitulation. These ideas originated in a nonevolutionary (creationist) context and only later were given evolutionary interpretations. All the morphologists used the similarities of embryos in sorting out homologies and establishing their archetypes. Louis Agassiz was a creationist champion of recapitulation while Ernst Haeckel gave it an evolutionary interpretation. Pandas' Footnote 4 refers to Haeckel (1866). Clearly, recapitulation is not exclusively an evolutionary concept as implied by the title of this section!
Recapitulation has been shown to be untenable by 20th century embryologists and the similarity of embryos is explained differently, i.e. most evolutionary changes in development occur in later stages. The early stages are so intertwined with webs of induction coordinating these initial stages that it is almost impossible to make changes without destroying the system. Thus the early stages, for example, are hardly different from those of the ancestral fishes. (See the supplement Embryos And Evolution for more details, including vestigial organs).
Pandas is thus more than 60 years out of date(!) when it insists on discussing the embryological evidence in terms of recapitulation. Human embryos do develop a tail that in the 5th week is one sixth the length of the embryo and contains a number of somites (incipient vertebrae). During the next four weeks, it disappears from external view, partly through actual regression. The coccyx, the remnant of the tail, recedes to a higher position in relation to the buttocks. The coccygeal fovea, or postanal pit, of a newborn marks the site where the coccyx disappeared below the surface. (Arey, 1946, p. 184; Gould, 1982 for a photo). Why should an intelligent designer give the human embryo a temporary tail to make a coccyx which then has to move to the proper place to be the point of attachment for some muscles?
Isn't it strange that an intelligent designer would provide the human embryo with three different sets of excretory organs?
Pandas' discussion of The Skeleton: A Hard Case for Recapitulation rests on incorrect data. The earliest ostracoderm fishes had heavy and complete coat of dermal bony armor. The dermal or membrane bones of modern forms are not preceded by cartilage but develop directly within blastemal (i.e. mesenchymal) sheets (Arey, 1946, p. 365). Also none of these earliest fossil fishes show signs of endoskeletons. They may have had cartilaginous endoskeletons, but cartilage normally doesn't fossilize. Only by the upper Silurian period do we find ossified internal skeletons (Carroll, 1988, p. 35). Thus the fossil evidence is not at odds with what one might have expected on the basis of recapitulation.
The Intelligent Design Interpretation Of Homology
Pandas' conclusion (p. 133) that homology appears to be an unreliable concept, and that the evidence for evolution from comparative anatomy and embryology is weak and misleading is totally unwarranted and erroneous! Furthermore, if homology is an unreliable and essentially worthless concept, why do the design proponents embrace it and boast that it was originated long before Darwin (p. 133).
The idea of a "small vocabulary of forms" used over and over again by the designers is a totally ad hoc hypothesis that does not explain the "grand design" discovered by the early anatomists. True, designing by random selections from a bag of tricks would not only leave in disarray our efforts to trace evolutionary relationships, (which they aren't) but would make a hierarchic classification impossible (which hasn't happened either.) How does this concept explain why the panda wasn't given a real thumb? or enzymes to digest bamboo? or why the wings of pterosaurs, birds and bats are different? Or why the enzyme cytochrome C (see chapter 6) isn't identical in all forms? When you try to use it, it becomes clear that it doesn't explain the way things really are.
Classifications Mainly Unchanged
It is true that classification has not much changed by the acceptance of evolution. Both the evolutionary and pre-evolutionary taxonomists based their classifications on perceived homologies. Many of the latter recognized the same homologies and analogies that evolutionists do. It is just the interpretation of those homologies that differ—indicating common descent instead of the features of a type or archetype. Thus Darwin said, ". ..systematists will be able to pursue their labours as at present." (Darwin, 1968, p. 455).
Distinguishing between similarities due to homology, convergence, and parallelism, guided by the principles of conservation of ancestral characters and irreversibility—"These are the most important principles that validate and guide phylogenetic grouping by morphological characters-in-common, the modern equivalent of archetypal grouping, sometimes so similar to the latter in appearance as to be mistaken for it but fundamentally different in principle." (Simpson 1945, p. 11).
Comparing the first edition of Gegenbaur's great textbook of comparative zoology (published in 1859 just before the Origin) with the second edition, published 11 years later reveals remarkably little difference except that terms like "morphological type" or "archetype" were replaced by "common ancestor" (Mayr, 1982).
Cladistics
The Pandas references to cladists (pp. 127, 133) are misleading. Cladistics was introduced to biologists by the German entomologist Willi Hennig in a 1950 book. His goal was an objective method for reconstructing phylogenies and achieving Darwin's prediction that classifications should become ‘genealogies.'. It was a response to the widespread habit, among biologists and palaeontologist, of classifying organisms on the basis of heterogeneous criteria (morphological, ethological) and according to their overall resemblance and subjectively determined degree of divergence. Previous classifications often reflected the importance or weight given somewhat subjectively to various characters by the taxonomist.
Cladists attempt to discover evolutionary branching sequences by identifying shared derived characters—homologous similarities uniquely present at a branching point. (Janvier, 1984; Luria et al. 1981. pp. 679-682; Patterson, 1978, pp. 125-127) All taxonomists rely on similarities and differences among organisms! (Luria et al, p. 670 fol.) Some cladists (‘transformed' or ‘pattern' cladists) try to make their methods and procedures independent of evolutionary theory so that the patterns they find (cladograms) can be used as independent and falsifiable tests of evolutionary hypotheses, not because they want to give nonevolutionary interpretations of them! They were originally prompted to make cladistics independent of evolution because of their misinterpretation of statements made by the philosopher of science, Karl Popper (and later repudiated by Popper) that Darwinism was untestable. See Charig, 1982 for a lucid account of the philosophy of transformed cladistics and Sonleitner, 1896 for the views of Karl Popper. Also, cladistic methodology is designed to work with extant living forms. If a fossil form is included in the analysis, it can only be accommodated as a sister taxon and not as an ancestral form. This may be the basis for the quotation from Patterson (a pattern cladist) at the end of excursion Chapter 4.
References:
Archer, M. 1984. Origins and early radiations of marsupials. In: Archer, M and G. Clayton. Vertebrate Zoogeography and Evolution in Australasia. Hesperian Press. pp. 585-626.
Arey, L. B. 1946. Developmental Anatomy: A Textbook and Laboratory Manual of Embryology. 5th Edition. W. B. Saunders Company.
Bergamini, D. 1964. The land and wildlife of Australia (Life Nature Library). Time Inc. N. Y.
Carroll, R. L. 1988. Vertebrate Palaeontology and Evolution. W. H. Freeman and Co. N.Y.
Charig, A. J. 1982. Systematics in Biology: A Fundamental Comparison of Some Major Schools of Thought. In: Joysey, K. A. and A. E. Friday (Editors). Problems of Phylogenetic Reconstruction. Academic Press, N. Y. pp. 363-440.
Darwin, C. 1968. The Origin of Species. (reprint of 1859 Edition) Penguin Books
Davenport, G. (Editor) 1983. The Intelligence of Louis Agassiz: A specimen book of scientific writing. Greenwood Press, Publishers. Westport, Connecticut
Davis, D. D. 1964. The Giant Panda: A morphological study of evolutionary mechanisms. Fieldiana. Zoology Memoir 3. Chicago Natural History Museum.
Denton, M. 1986. Evolution: A Theory in Crisis. Adler and Adler.
Forey, P. 1982. Neontological Analysis Versus Paleontological Stories. In: Joysey, K. A. and A. E. Friday (Editors). Problems of Phylogenetic Reconstruction. Academic Press, N. Y. pp. 119-157.
Goodrich, E. S. 1958. Studies on the structure and development of vertebrates. 2 vols. (reprint of an 1895 book). Dover Publications, Inc.
Gould, S. J. 1978. The Panda's Peculiar Thumb. Natural History 87(9): 20-30. (November).
Gould, S. J. 1980. The Panda's Thumb: More reflections in Natural History. W. W. Norton & Co. N. Y.
Gould, S. J. 1982. Fascinating tails. Discover 3(9):40-41 (September).
Gould, S. J. 1985. A clock of evolution. Natural History (April): 12-25.
Gould, S. J. 1986a. Fuzzy Wuzzy was a bear. Andy Panda, too. Discover 7(2): 40-48 (February).
Gould, S. J. 1986b. Archetype and Adaptation. Natural History (October): 16-27).
Gould, S. J. 1988. The heart of terminology. Natural History (February): 24-30.
Gould, S. J. 1990. An Earful of Jaw. Natural History (March): 12-23.
Haeckel, E. 1866. General Morphology of Organisms. Georg Reimer, Berlin.
Horner, J. R. and J. Gorman. 1988. Digging Dinosaurs. Workman Pub. Co. N. Y. pp. 163-165.
Janvier, P. 1984. Cladistics: Theory, purpose, and evolutionary implications. In Pollard, J. W. (Editor). Evolutionary theory: Paths into the Future. pp. 39-75.
Luria, S. E., S. J. Gould and S. Singer. 1981. A View of Life. The Benjamin/Cummings Pub. Co.
Keast, A. 1982. The thylacine (Thylacinidae, Marsupialia): How good a pursuit carnivore? In: Archer, M. (Editor). Carnivorous Marsupials (2 volumes). Royal Zoological Society of New South Wales. pp. 675-684.
Mayr, E. 1963. Animal Species and Evolution. The Belknap Press of Harvard Univ. Press.
Mayr, E. 1982. The Growth of Biological Thought: Diversity, Evolution and Inheritance. Belknap Press of Harvard University Press. (see pp. 455-469).
Mayr, E. 1986. Uncertainly in science: Is the giant panda a bear or raccoon? Nature 232:769-771.
O'Brien, S. J. 1987. The Ancestry of the Giant Panda. Scientific American 257(5): 102-107 (November).
O'Brien, S. J., W. G. Nash, D. E. Wildt, M. E. Bush and R. E. Benveniste. 1985. A molecular solution to the riddle of the giant panda's phylogeny. Nature 317: 140-144.
Park, A. 1985. Is this toothy relic still on the prowl in Tasmania's wilds? Smithsonian 16(5): 117-130 (August).
Patterson, C. 1978. Evolution. British Museum (Natural History) Cornell Univ. Press.
Patterson, C. 1981. Significance of fossils in determining evolutionary relationships. Annual Review of Ecology and Systematics 12: 195-223.
Sarich, V., J. M. Lowenstein and B. J. Richardson. 1982. Phylogenetic relationships of the thylacine (Thylacinus cynocephalus, Marsupialia) as reflected in Comparative Serology. In: Archer, M. (Editor). Carnivorous Marsupials (2 volumes). Royal Zoological Society of New South Wales. pp. 707-709.
Schaller, G. B., T. Qitao, K. G. Johnson, W. Xiaoming, S. Heming and H. Jinchu. 1989. The Feeding Ecology of Giant Pandas and Asiatic Black Bears in the Tangjiahe Reserve, China. In: Gittleman, J. L. Carnivore Behavior, Ecology, and Evolution. Comstock Publishing Associates. pp. 212-241.
Simpson, G. G. 1945. The Principles of Classification and a Classification of Mammals. Bulletin of the American Museum of Natural History 85:1-350.
Simpson, G. G. 1961. Principles of Animal Taxonomy. Columbia Univ. Press. N. Y.
Smith, M. 1982. Review of the thylacine (Marsupialia, Thylacinidae). In: Archer, M. (editor). Carnivorous Marsupials (2 volumes). Royal Zoological Society of New South Wales. pp. 237-253.
Sonleitner, F. J. 1986. What Did Karl Popper Really Say About Evolution? Creation/Evolution 6(2):9-14
Walker, E. P. 1975. Mammals of the World, 3rd Ed. (2 volumes). Johns Hopkins University Press.
Wayne, R. K., R. E. Benveniste, D. N. Janczewski, and S. J. O'Brien. 1989. Molecular and Biochemical Evolution of the Carnivora. In: Gittleman, J. L. Carnivore Behavior, Ecology, and Evolution. Comstock Publishing Associates. pp. 465-494.
Wozencraft, W. C. 1989a. The Phylogeny of the Recent Carnivora. In: Gittleman, J. L. (Editor). Carnivore Behavior, Ecology, and Evolution. Comstock Publishing Associates. pp. 495-535.
Wozencraft, W. C. 1989b. Classification of the Recent Carnivora. In: Gittleman, J. L. (Editor). Carnivore Behavior, Ecology, and Evolution. Comstock Publishing Associates. pp. 569-593.
(from Frank Sonleitner's critique of Of Pandas and People)
