Last week I made a case that origins-of-life research doesn’t usually fall under the evolution umbrella. I offered my analogy that the first spark of life was a bit like a baton hand-off from chemical evolution to biological evolution. Today, I’ll get into some aspects of the topic that tend to evoke the most, well, heated and let’s say spirited discussion.
Perhaps the single biggest lightning rod is that chemical evolution is the quest to understand how non-life could have come to form life. Aside from the obvious conflicts with a literal interpretation of many creation stories, there’s also the suggestion that life coming from non-life would violate the law of biogenesis. What’s next? Maggots 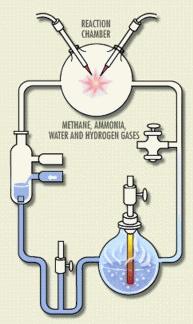 forming spontaneously from rotting meat? (Ew.) Relax. While superficially the two situations seem similar, they really aren’t. That maggots can’t spring from a sealed-off hunk of steak in your kitchen does not disprove the idea that under the conditions of early Earth, organic molecules could have formed a living cell. And of course there’s plenty of research, going back to the classic Miller-Urey experiment, which suggests that the conditions of early Earth were suitable for the formation of complex organic molecules.
forming spontaneously from rotting meat? (Ew.) Relax. While superficially the two situations seem similar, they really aren’t. That maggots can’t spring from a sealed-off hunk of steak in your kitchen does not disprove the idea that under the conditions of early Earth, organic molecules could have formed a living cell. And of course there’s plenty of research, going back to the classic Miller-Urey experiment, which suggests that the conditions of early Earth were suitable for the formation of complex organic molecules.
Another common objection to chemical evolution is that it seems so unlikely! I mean, random chemicals just kind of come together to form nucleic acids and a cell membrane? What are the chances? Some naysayers have even tried to put a number on it—the Institute for Creation Research’s textbook Scientific Creationism (1974), for example, estimated that the probability would have been less than 1 in 1053. But, as NCSE’s Genie Scott and Glenn Branch point out in their 2012 article on this topic, such estimations usually fall into one or more traps, assuming this process would have been entirely random, for example, or that the first cell would have resembled a modern one.
Another favorite drum to beat on this topic is that the Miller-Urey experiment was wrong, all wrong! Well, it wasn’t. It’s true that the mixtures of gases used originally weren’t perfect in terms of replicating Earth’s early atmosphere, but 1) the experiments have been replicated with the right mixture and the same results were obtained, and 2) not all origins-of-life research rests on the Miller-Urey experiment anyway. It’s more than fifty years since the experiment, and plenty of smart scientists have been working on the origin of life in that time!
I’m reminded of the crux of “intelligent design” arguments, that life is all so complicated, so perfect that it can’t be explained by natural forces alone. And that always frustrates me because it’s such a cop-out. Just because we can’t at this moment explain everything we should give up the quest and ascribe it to a deus ex machina? What kind of lesson does that impart to our kids? By parity of reasoning, I guess a kindergarten teacher should be okay with an excuse from a five-year-old along the lines of, “I don’t get quadratic equations, so clearly all math is too complicated to be understood, so I’m going to abstain from all math-related activities.” (This five-year-old has very advanced language skills, however.)
So what should a teacher do? I’m sure that some of you have been tempted to say, or have even said, that it’s okay for students to think that a supernatural force created the first life, as long as they accept that evolution took over from there. But I’d urge you not to do that. Besides the obvious issue with crossing the line into non-science with your students, saying that kind of a thing effectively slams a door shut in their faces. It’s like suggesting that they don’t need to worry about the science before cells if they don’t want to—but why shouldn’t they want to? It’s fascinating stuff! Sure, there isn’t currently a detailed and generally accepted scenario for the origin of life, but that doesn’t make it okay to invoke the supernatural. Just because we don’t know now doesn’t mean we’ll never know.
I took a class on the social impact of scientific revolutions when I was in college. The professor was a science historian and taught his courses using images. Every lecture was based around a slide show. The one image that has stuck with me more than any other was a medieval-like (but actually nineteenth-century) image of a man reaching through the last celestial sphere in the standard Ptolemaic or Copernican concentric model of the universe. Some have interpreted the image to represent the sixteenth-century Italian monk Giordano Bruno, who questioned the idea of fixed celestial spheres. If the model is correct, Bruno wondered, then what’s beyond the last sphere? What I love about the image is that it displays such an obvious question. How could anyone in the  sixteenth century not wonder what was beyond the edge of the universe? In my opinion, research into the origins of life is akin to wondering what happens if you try reach beyond the sphere of stars. Of course we should ask! Of course we should try to find out how life began! And to go in search of these answers doesn’t take away any mysteries. If anything, it opens more up. Isn’t science grand?
sixteenth century not wonder what was beyond the edge of the universe? In my opinion, research into the origins of life is akin to wondering what happens if you try reach beyond the sphere of stars. Of course we should ask! Of course we should try to find out how life began! And to go in search of these answers doesn’t take away any mysteries. If anything, it opens more up. Isn’t science grand?
Are you a teacher and want to tell us about an amazing free resource? Do you have an idea for a future Misconception Monday or other post? See some good or bad examples of science communication lately? Drop me an email or shoot me a tweet <at>keeps3.

