I want to start this week’s entry by saying that I really hadn’t intended this topic to take up three posts! It’s just that I kept adding and adding to make it all make more sense and before I knew it, I had 3000 words on dating fossils! Words fly when you’re geeking out…
So, last time, we discussed the basics of radiometric dating and ended with a quick and dirty example of how a parent:daughter isotope ratio can be used to find the age of a sample. I skipped some details on purpose, but the foundational principles to these methods are really as easy as I explained. So how come I started this whole thing by saying that it’s actually not possible to date fossils directly? Why can’t you just measure the ratios and move on? Because although radiometric dating will almost always provide an answer, in most cases, unless you’re applying the methods to an igneous rock, it won’t answer the question you’re asking.
See, the clock for radioactive elements starts at crystallization. Crystallization happens in the formation of both igneous and metamorphic rocks. For igneous rocks, it occurs as the magma or lava cools to form minerals, and for metamorphic rocks, it occurs when heat and/or pressure alter the composition of pre-existing minerals. As the rock cools, there comes a point, called the closing temperature, when parent and daughter isotopes can no longer diffuse into or out of the rock system—at that point, the clock is set. (It’s worth mentioning that this temperature varies by rock type and by isotope! So it’s not like there is one magic temperature that locks these things in. It’s all a bit mathy, but the good thing is, people have figured it out and can account for it no matter the rock type or the radiometric method being used.)
But, when it comes to fossils, we’re primarily interested in sedimentary rock layers, which, you’ll recall, form from pre-existing rock material. So if you try to run radiometric dating on a sedimentary rock, you won’t determine the age of that rock—that is, when the sediments were compacted and cemented to form a new layer. Instead, you’ll determine how long ago the parent rock formed—not very helpful. And when you consider that sedimentary rocks are where fossils are found, you might despair of the prospect of using radiometric dating to ascertain the age of your favorite fossil.
But wait! What about carbon-14? Everyone is always talking about carbon-14! Can’t you use that? Not really. Carbon-14 is a radioactive form of carbon that is widely used in radiometric dating. Very occasionally, there may be some remaining organic material in a fossil that could, hypothetically, be dated using carbon-14. However, there is a problem, a really big one: Its half-life is only 5730 years. It might not seem like it, but that is a really short half-life, and Ea rth’s history is really really long. Carbon-14 is basically useless if what you’re interested in is more than 50,000 years old—which, for pretty much all paleontologists, includes just about everything. A notable exception are the absolutely awesome mammoth specimens that are very datable by C-14 and full of perfectly preserved organic material.
rth’s history is really really long. Carbon-14 is basically useless if what you’re interested in is more than 50,000 years old—which, for pretty much all paleontologists, includes just about everything. A notable exception are the absolutely awesome mammoth specimens that are very datable by C-14 and full of perfectly preserved organic material.
So, in sum: C-14 is great if you’re Indiana Jones (who, for once and for all, was an archaeologist, not a paleontologist, okay? Big difference. For one thing, paleontologists get chased by super smart Velociraptors, not antiques-looting Nazis.). But for the rest of us, C-14 is not very helpful.
How, then, can radioactive dating help us? By helping us to date igneous rocks, of course! Radioactive dating also helps to date metamorphic rocks, but since metamorphosis happens to preexisting rocks, those dates aren’t very useful to know because they don’t help you to put anything in order. Did the rock metamorphose before or after the sedimentary rock was laid down on top of it? You wouldn’t know—and this is all about knowing just that. Yes, we’re back to relative dating principles—but this time, with numbers! And that makes all the difference because once we know the absolute dates of the relevant igneous rocks, we can parlay that information into giving us a range for the possible absolute dates of the fossils. Let’s revisit the diagram from two weeks ago to see how it works.
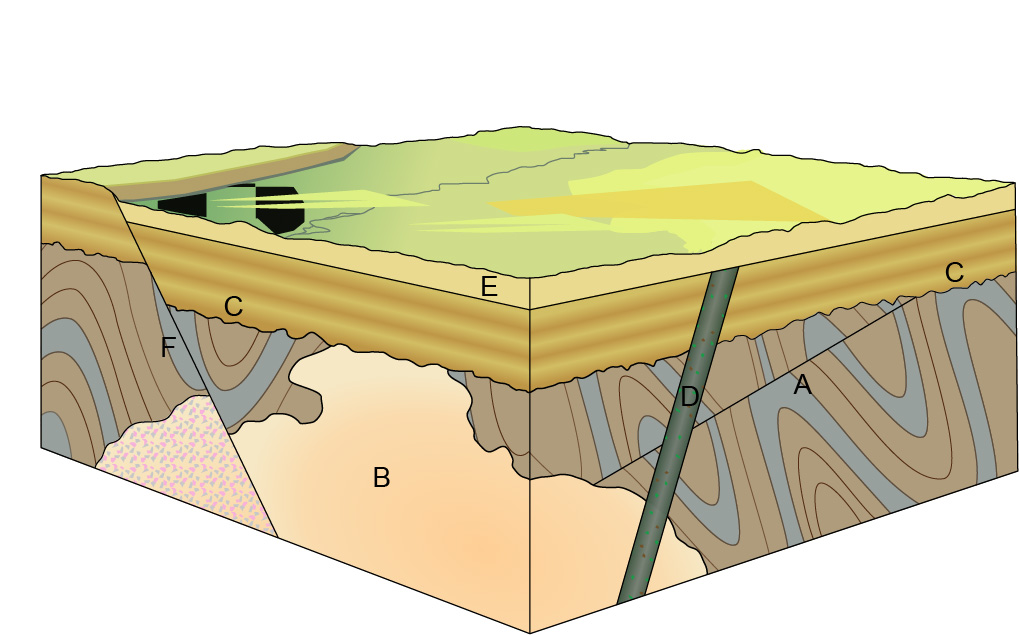
I asked in the caption to this figure, “How do you know C happened before D?” The answer was that D cut across layer C. If you’re familiar with the colors and patterns used in geologic diagrams like this, you probably know that D is an igneous intrusion and C is a layer of sedimentary rock. So we know that C came before D—but because D is igneous, we can put a date on it (now I have a Beyoncé song in my head, great…). If radiometric dating determines the age of that layer to be 150 million years, then suddenly we know that C is older than that. Hooray! But wait, you say, that gives it an age range on the order of billions of years: is that really so helpful? But 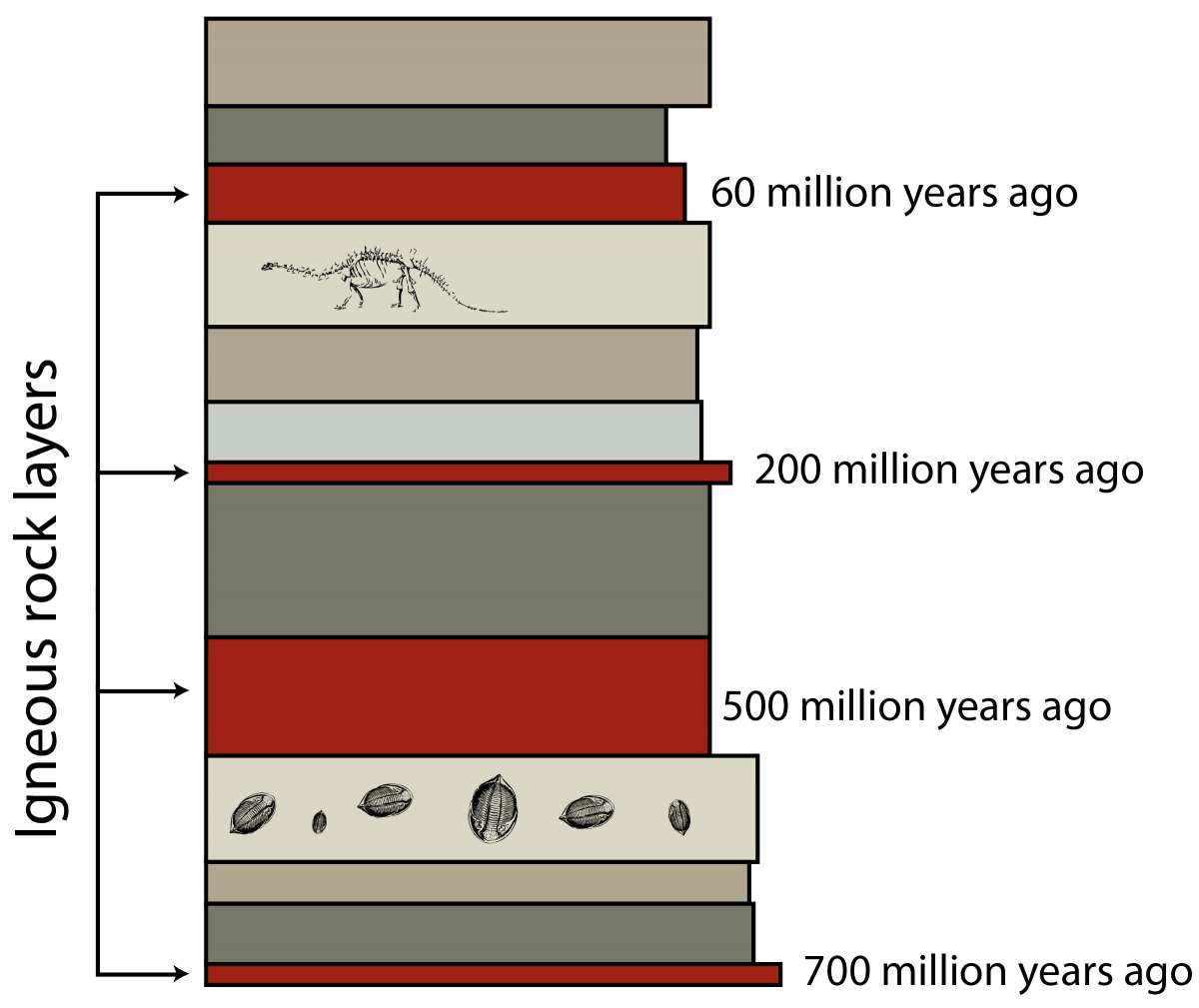 remember! There are rocks all over the world that can, with the help of fossil assemblages, be correlated. So maybe somewhere else, a correlated bed of this sedimentary rock sits on top of a layer of igneous rock that is 160 million years old—then our temporal window for C has shrunk to 10 million years. Not too bad in the grand scheme of things … but with a few more localities thrown into the mix, geologists can narrow it down further. It works remarkably well.
remember! There are rocks all over the world that can, with the help of fossil assemblages, be correlated. So maybe somewhere else, a correlated bed of this sedimentary rock sits on top of a layer of igneous rock that is 160 million years old—then our temporal window for C has shrunk to 10 million years. Not too bad in the grand scheme of things … but with a few more localities thrown into the mix, geologists can narrow it down further. It works remarkably well.
Unless you teach high school or college Earth science or perhaps chemistry, most of this information may fall outside your curriculum. However, that doesn’t mean that you can’t help to push against the misconception that all fossils are dated directly. Most obviously, you yourself can be careful not to suggest that fossils are dated directly. Further, if a student asks how we know how old fossils are, or suggests carbon dating a dinosaur, you can take the time to explain the information, or send  them on a guided webquest to find for themselves. There’s all kinds of material out there appropriate for even the youngest grades. Just please, if a student talks about how they want to be a paleontologist like Indiana Jones, correct them there and then.
them on a guided webquest to find for themselves. There’s all kinds of material out there appropriate for even the youngest grades. Just please, if a student talks about how they want to be a paleontologist like Indiana Jones, correct them there and then.
Have an idea for a future Misconception Monday or other post? See some good or bad examples of science communication lately? Drop me an email or shoot me a tweet <at>keeps3.

